Infectious diseases caused by pathogenic microorganisms are currently the second leading cause of death worldwide, particularly in low- and middle-income nations. Accurate and rapid diagnosis is essential to manage the spread of this infectious disease and can help with better clinical decision-making as well as disease control and prevention. However, current testing methods, such as culture- and nucleic-acid-based techniques, are often unavailable in the developing world due to resource limitations.

This study is featured as Back Cover of Lab on a Chip.
To address this issue, lateral flow assays (LFAs), which are a type of paper-based device that can perform rapid immunoassays via capillary action of the reagents, have been proposed for pathogen diagnosis in developing countries as they are low-cost, relatively simple to manufacture, do not require power, and can be made widely available. However, LFAs generally possess limited sensitivity and can only provide qualitative detection results. To enhance the sensitivity and enable multi-step and quantitative detection, three dimensional (3D) microfluidic paper-based analytical devices (µPADs) have been developed, enabling sequential steps of assays to be easily performed by stacking and folding multiple fluidic layers from a single sheet of paper. This design provides 3D µPADs with superior advantages over LFAs in terms of sensitivity, multiplexity, sample consumption, and quantitative analysis. However, external instruments and electricity are still required to complete the assays.
To address these obstacles, we demonstrate an electricity- and external instrument-free sensor for infectious disease diagnosis based on a 3D µPAD. By performing a colorimetric enzyme-linked immunosorbent assay (ELISA) on the µPAD, the presence of the target analyte can be converted to a dark blue color that is visible to the naked eye, preventing the need for an excitation light source or smart phone. The 3D µPAD adopts the principle of origami (paper folding), enabling the multi-step ELISA test to be conducted by simply folding and sliding the paper-based components of the µPAD. Additionally, we have integrated an electricity-free “timer” function into the µPAD to indicate the accurate timing for each ELISA step (3D-tPADs). To incorporate an automated timer function in the device, we create different fluidic path volumes that delivers the excess washing buffer from the absorbent pads to the dye wells, which are pre-treated with dried green food dye, thus coloring the solution and turning the indication wells green. This color signals the time at which different steps in the assay should be performed. Moreover, by pre-treating the paper substrate with dried sucrose and pullulan, we are able to control the viscosity, and therefore the flow rate, of the washing buffer to ensure it transfers the dye to the indication wells at the appropriate times for conducting different steps of the test.
To verify the feasibility of the sensor for infectious disease diagnosis, we detect blood plasma containing HIV type 1(HIV-1) p24 antigen, which is recognized as a virological biomarker in early HIV detection. As a result, a detection limit as low as 0.03 ng/mL can be obtained in 10 min. Throughout the whole detection process, no electricity nor external equipment is required, enabling the sensor to be applied for on-site infectious disease diagnosis and extending its usage to resource-limited areas and the developing world.
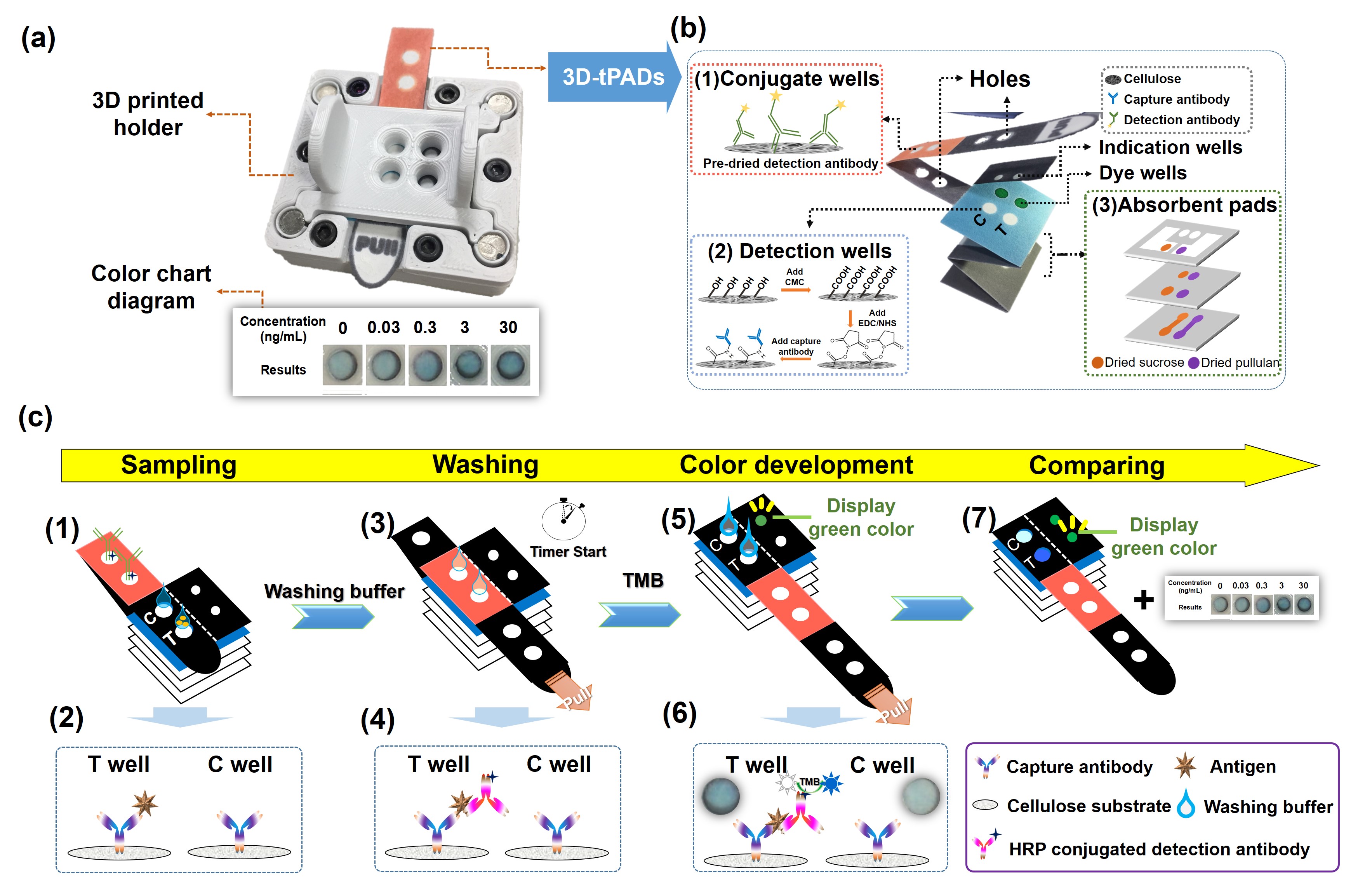
(a) Images of the components of the proposed infectious disease sensor, including a 3D-tPADs, 3D-printed holder, and color chart diagram. (b) The structure of the proposed 3D-tPADs. (c) The operation procedure of the ELISA test on the proposed 3D-tPADs.
This work has been published and featured on the Back Cover on Lab on a Chip, and it is selected and collected in the Lab on a Chip HOT Articles 2021. The team would like to acknowledge and appreciate the financial support from the Ministry of Science and Technology (MOST) and NTU’s Higher Education Sprout Project.
Contact: Prof. Chien-Fu Chen
This email address is being protected from spambots. You need JavaScript enabled to view it.
More information:
“An electricity- and instrument-free infectious disease sensor based on a 3D origami paper-based analytical device,” Lab on a Chip
http://xlink.rsc.org/?DOI=D1LC00079A
