Mycobacterium tuberculosis, an aerobic and acid-resistant microbe, primarily affects the lungs and can spread to other organs through droplets and airborne particles. This leads to forms of tuberculosis outside the lungs, including pleural, skeletal, central nervous system, and abdominal tuberculosis, making it among the world's most lethal infectious diseases. The World Health Organization's 2022 annual report revealed that around 10.6 million people globally were diagnosed with tuberculosis, and about 1.4 million died in 2021. Most cases were concentrated in low-resource areas, making it one of the top ten causes of death and one of the world's most severe and urgent public health problems.

Traditional methods for diagnosing tuberculosis, such as sputum smear microscopy, cell culture, and molecular-based tests, play a crucial role in healthcare. These diagnostic techniques are indispensable for accurately detecting TB, but they demand specialized equipment, consistent electricity supply, and skilled professionals to perform the procedures effectively. Unfortunately, in many remote or developing areas, the availability of such medical resources is severely limited. This scarcity hinders the TB diagnosis, exacerbating the challenge of controlling the disease in these regions.
In this study, we illustrate that palladium–platinum bimetallic nanoparticles (Pd@Pt NPs) as a nanozyme, combined with a multi-layer paper-based analytical device and DNA hybridization, effectively detect Mycobacterium tuberculosis. This nanozyme exhibits peroxidase-like activity that enhances the oxidation rate of the substrate. When compared to horseradish peroxidase, widely employed in traditional detection, the Michaelis constants for Pd@Pt NPs are significantly lower, being fourteen and seventeen times less for 3,3',5,5’-tetramethylbenzidine and H2O2, respectively. To verify the catalytic efficiency of Pd@Pt NPs, our study conducts the molecular diagnosis of Mycobacterium tuberculosis. We selected the IS6110 fragment as the target DNA, dividing the complementary sequences into capture and reporter DNA. They were modified on paper and Pd@Pt NPs to detect Mycobacterium tuberculosis on the paper-based analytical device. With the method mentioned above, the target DNA can be detected within 15 minutes, exhibiting a linear range from 0.75 to 10 nM and achieving a detection limit of 0.216 nM. These results demonstrate that the introduced platform (a DNA–nanozyme integrated paper-based analytical device, dnPAD) offers sensitive and immediate infection diagnosis in regions with limited medical facilities.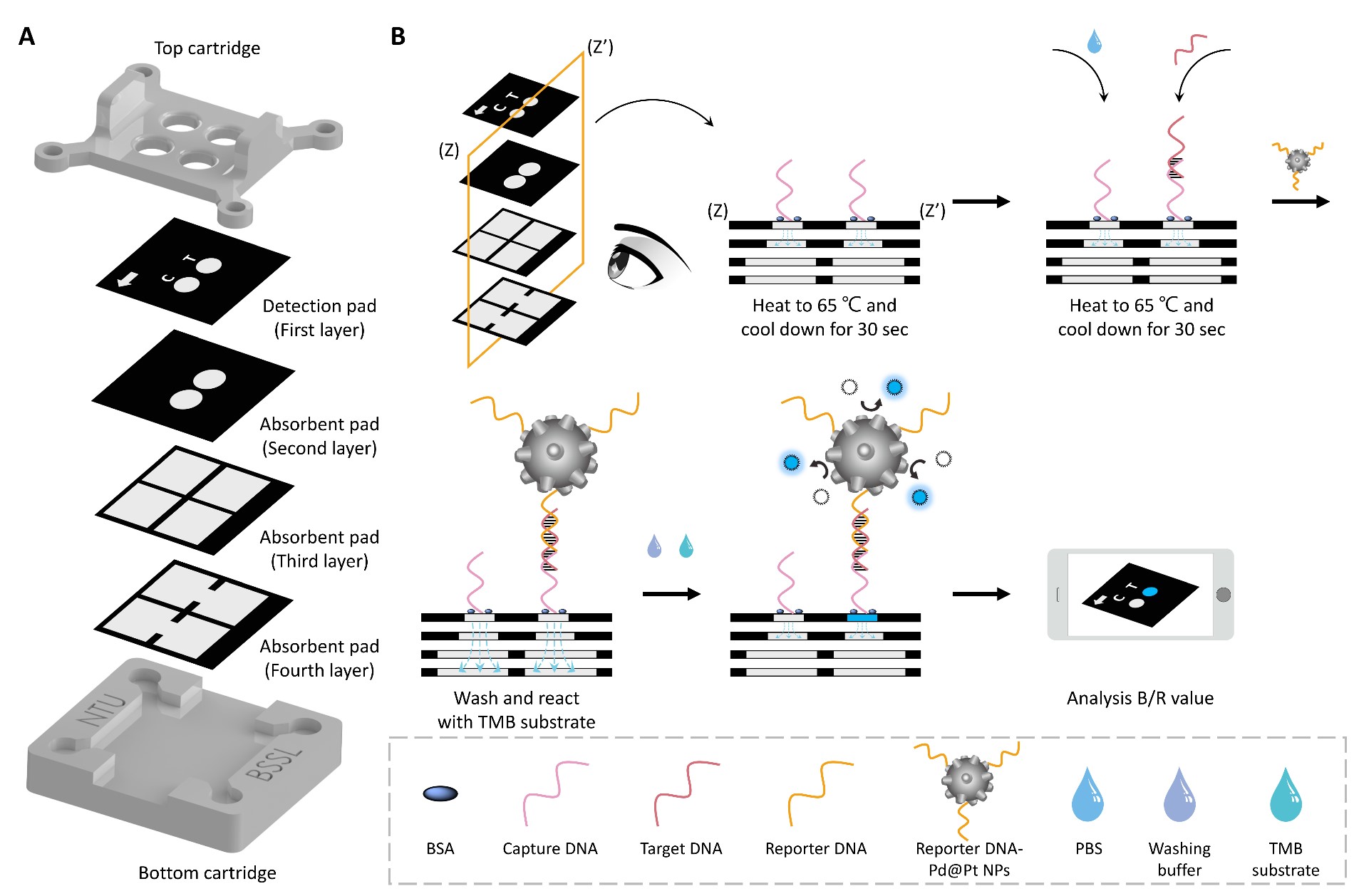
An overview mechanism of the experimental operation procedure for Mycobacterium tuberculosis IS6110 detection. (A) A schematic diagram of the proposed dnPAD, including the detection and absorbent pads composed of the wax-printed paper sheets, sandwiched between two 3D-printed cartridges. (B) The operation procedure of the DNA hybridization test on the dnPAD, including the addition of reporter DNA-Pd@Pt NPs, washing, and catalyzation of the TMB substrate by Pd@Pt NPs.
This work has been published and featured on the Inside Front Cover of Nanoscale. The team would like to acknowledge and appreciate the financial support from the National Science and Technology Council and the Higher Education Sprout Program at National Taiwan University, Taiwan.
Contact: Prof. Chien-Fu Chen
This email address is being protected from spambots. You need JavaScript enabled to view it.
More information:
“Diagnosis of Mycobacterium tuberculosis using palladium–platinum bimetallic nanoparticles combined with paper-based analytical devices” Nanoscale, 2024, 16, 5988.
https://pubs.rsc.org/en/content/articlelanding/2024/nr/d3nr05508f
