Prosthetic-joint replacements are used to improve patients’ quality-of-life by providing symptom relief, increasing mobility, and restoring joint function. In general, these surgeries are safe and cost-effective, however, prosthetic joint infection (PJI) remains one of the most common causes of hip revisions and knee arthroplasties. Incorrect diagnosis can lead to underestimation of early PJIs, as well as delayed infections, which are even more difficult to treat.
In the effort to combat PJIs, there are many assays available that can help identify possible infections. Among them, lateral flow immunoassays (LFIAs) are a leading technology in point-of-care diagnostics due to their simplicity, speed, and low cost. Point-of-care diagnostic devices are not generally intended to replace more sophisticated laboratory tests, but rather provide a means of rapid initial screening in non-laboratory settings.
LFIAs are simple diagnostic devices that combine chromatography and nanoparticle-labeled immunoassay technology, in which an analyte passes through multiple membranes, including a sample “pad” for sample loading, a conjugate pad for specifically labeling the analyte with the functionalized gold nanoparticles conjugated with antibodies, a nitrocellulose-based test pad to produce the visible result of an immobilized captured reagent, and the absorbance pad for controlling the overall loading volume. However, the detection of individual biomarkers by LFIAs has been shown to lack the specificity and sensitivity necessary for diagnosis in clinical settings. Therefore multiplexing (i.e., the detection of multiple biomarkers) is a critical need for increasing diagnostic efficiency and accuracy.
In our study, ”Development of a multiplex and sensitive lateral flow immunoassay for the diagnosis of periprosthetic joint infection,” we demonstrate a multiplex LFIA platform constructed from paper patterned with a gold-nanoparticle-based immunoassay for the detection of PJI biomarkers in as little as 20 min. Specifically, we use this design to detect alpha-defensin and C-reactive protein, which are sampled from the synovial fluid of patients undergoing revision arthroplasty for septic or aseptic failure. To achieve signal amplification without additional procedures or the need for complex fabrication, we have developed a simple method to “stack” the configuration of the assay’s membranes in a manner that adds an additional membrane between the conjugation pad and test pad to the conventional gold-nanoparticle-based LFIA format. This design helps to accumulate the antibodies and antigens to extend their binding interactions to enhance the test’s detection sensitivity.
Using this platform and a specialized algorithm, we can correctly diagnose 90% of PJI cases using our multiplex LFIA device. This concept provides multiple, parallel protein measurements on the same specimen, which is valuable given the need to detect several biomarkers simultaneously to generate meaningful or conclusive information, the analysis of which could be further improved using artificial intelligence. In the future, we expect that our accessible and programmable multiplex LFIA platform could be easily utilized at any point of need within the healthcare system.
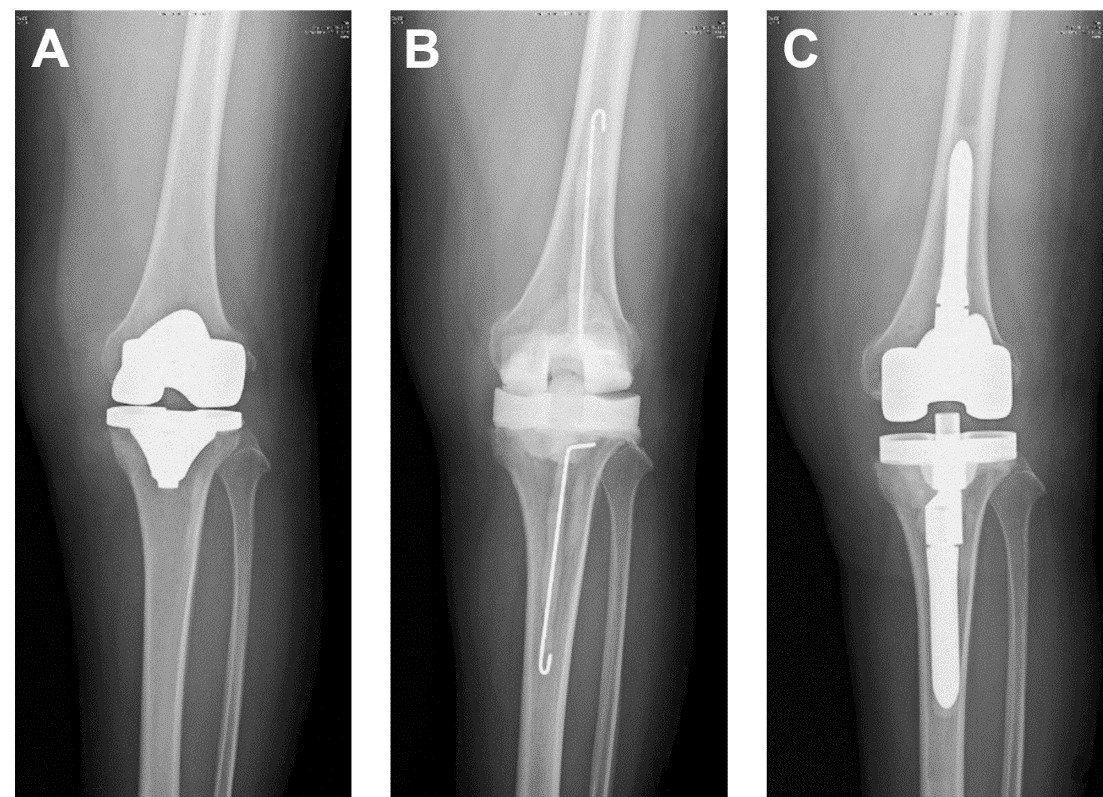
Representative radiographs of prosthetic joint infection (PJI). PJI diagnosis can be challenging and sometimes is based on clinical suspicion with no clinical, laboratory, or radiographic features that can confirm the diagnosis. Therefore, a point-of-care diagnostic device that can accurately diagnose PJI would be highly beneficial. In this case, (A) the image did not reveal PJI, but wound discharge indicated that the patient had developed PJI after a total knee replacement. (B) Metal prothesis was removed, soft tissues were debrided, and temporary antibiotic bone cement spacer was inserted. (C) Permanent total knee replacement was inserted after well-controlled infection.
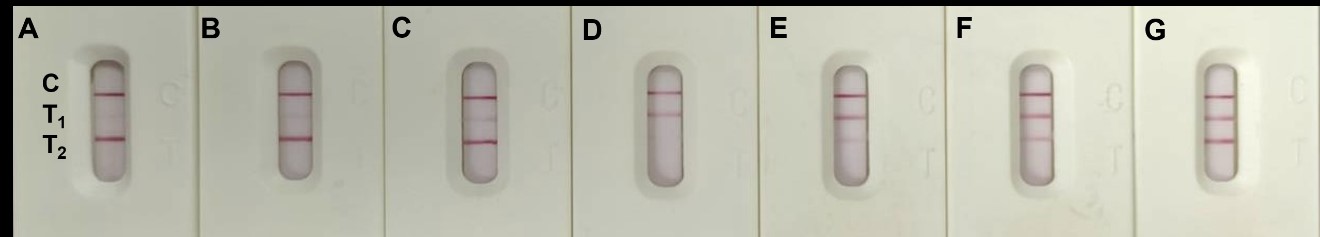
The test result images of our multiplex LFIA platform. Two different infection-related biomarkers were analyzed, the different concentrations from the positive T2 and gradually increase of T1 (A to C), and the positive T1 and gradually increase of T2 (D to G) were shown in this image.
Contact: Prof. Chien-Fu Chen
This email address is being protected from spambots. You need JavaScript enabled to view it.
Springer Nature Protocols and Methods Community
https://bit.ly/335XyNa
Scientific Reports
https://go.nature.com/2Xqs03a

